
Understanding Tritium
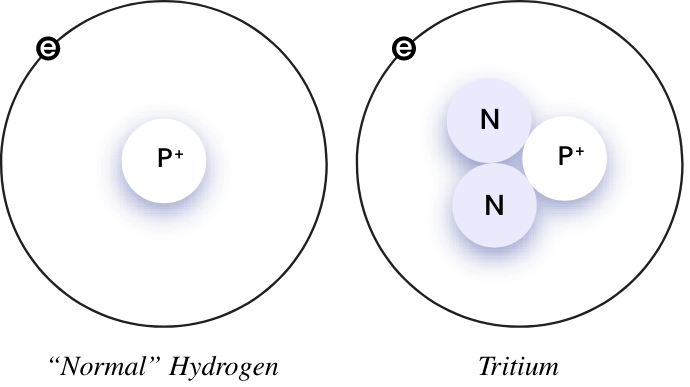
Tritium is a radioactive isotope of hydrogen, meaning it spontaneously and consistently emits radiation. It is distinguished by the presence of two neutrons and one proton in its nucleus, in contrast to the single proton with no accompanying neutrons found in ordinary hydrogen.
Widely recognized as a dependable source of self-sustaining energy, tritium has a diverse range of applications, including providing power for self-illuminating devices like exit signs and watch dials, as well as serving as fuel for specific types of nuclear reactors.
Where tritium is found depends on how it is created, as it can be produced in two primary ways: through natural processes in Earth’s upper atmosphere and artificially in nuclear reactors.
Natural tritium production occurs when cosmic rays interact with atmospheric gasses, creating tritium atoms. How artificial tritium is made is different, and instead involves irradiating lithium-6 with neutrons in a nuclear reactor—a process that converts some of the lithium-6 into tritium. Unlike other notorious radioactive isotopes like plutonium and uranium, tritium is relatively benign.

Radioactivity and Risks
The extra neutron in tritium’s nucleus makes it unstable, causing it to undergo a process called radioactive decay. During radioactive decay, one of the neutrons in tritium’s nucleus can transform into a proton through a process known as beta decay. This conversion of a neutron into a proton as tritium transitions to a more stable state (helium-3) results in the emission of a low-energy beta particle.
Tritium is a relatively weak beta radiation source, so it does not have enough energy to penetrate human skin. The only health risks from tritium come from ingesting, inhaling, or prolonged exposure. The naturally occurring radiation can be easily and effectively blocked through minimal protective coatings. The radioactive decay of tritium emits no gamma radiation, which is associated with more severe health risks.
Additionally, tritium has a short half-life, which means it decays relatively quickly, further reducing its long-term environmental impact. In summary, although tritium is radioactive, it is not inherently dangerous if basic precautions are taken.
Tritium Half-Life
Tritium’s half-life is a defining characteristic of its utility in various applications. Half-life refers to the time it takes for half of a radioactive substance to decay into a more stable state. At 12.3 years, tritium’s half-life is relatively short, meaning it decays relatively quickly compared to some other radioactive materials.
However, its short half-life is advantageous for practical applications, as it allows for predictable and controlled energy production that occurs independently of most environmental factors. This makes tritium uniquely capable of supplying power in extreme environments where traditional batteries that rely on volatile chemical reactions would not be able to function.
The low amounts of energy released as tritium decays allow for unique use cases, making it perfect for powering low-power devices and microelectronics through specially designed batteries, such as betavoltaic batteries, which are built to harness this reliable energy source.
NanoTritium™ Battery Applications
The robust, long-life, and reliable energy generated by NanoTritium™ batteries is ideal for powering low-power devices in several notable applications where traditional batteries struggle, including:

Space-Based Technology
As technology in space is often difficult or impossible to repair, batteries that can provide reliable power for multiple decades while resisting environmental challenges associated with space travel—such as intense vibration, extreme temperatures, and direct radiation—are highly desirable. NanoTritum™ batteries are perfectly suited for powering a variety of low-power devices in space, especially technology relevant to small satellites, such as image sensors or nuclear propulsion systems.

Defense Applications
The robust nature of NanoTritium™ batteries naturally lends itself for use in national security and defense applications where reliability is of utmost importance. City Labs’ batteries are especially well-suited for Communications Security (COMSEC) applications, such as providing resilient and long-lasting power for cryptographic devices that rely on battery power for security.
Medical Devices & Implants
Many implantable medical devices, including pacemakers and glucose sensors, rely on constant but low-power energy sources to provide their life-saving functions. Replacing these devices’ batteries is complicated and typically requires invasive surgery.
Increasingly, other medical devices and implants, such as artificial organs and prosthetic limbs, utilize some sort of low-energy electronics to function. Because NanoTritium™ batteries are reliable, long-lasting, and contamination-free, they are the perfect choice for powering low-power medical devices.
City Labs’ active efforts in this arena include a collaboration with Biotronik to develop the next generation of leadless pacemakers.

Sub-Sea Sensors & Actuators
Underwater exploration for both research and economic purposes frequently requires the use of sub-sea pressure sensors and actuators for critical mission functionality. NanoTritium™ batteries are well-suited for these applications, as they are built to withstand pressure, corrosion, water, and low temperatures.

Drilling
Downhole drilling requires substantial data collection to navigate beneath Earth’s surface safely and effectively. Logging and measurement data rely on advanced microelectronics to determine crucial drilling factors, such as ground porosity, pressure, hole direction, etc. Since these microelectronics are often subjected to harsh conditions like high pressure or intense vibrations, NanoTritium™ batteries offer a unique solution to powering drilling operations.

Additional Applications
City Labs is constantly striving to improve our NanoTritium™ capabilities and use cases, and we welcome your input.
If you have an exciting idea for powering low-power microelectronics of the future, we invite you to contact us.
What Are Betavoltaic Batteries?
Betavoltaic batteries are a unique class of nuclear batteries that harness the energy released during the decay of radioactive isotopes, such as tritium, to generate electricity. Unlike conventional batteries that rely on chemical reactions, betavoltaic batteries use the high-energy beta particles emitted by the decaying isotopes to initiate a flow of electrons. This creates a continuous source of electrical power for as long as the radioactive source material undergoes a decay process. Tritium’s 12.3-year half-life provides enough energy to power a betavoltaic battery for several decades.
City Labs’ NanoTritium™ Batteries
City Labs is the pioneering developer of NanoTritium™ technology, creating a customizable line of betavoltaic battery products that combine betavoltaic principles and tritium to make the world’s first commercially available nuclear battery.
The patented technology behind NanoTritium™ batteries provides a reliable and long-lasting power supply for various low-power microelectronic devices. These batteries produce electricity independently of temperature variations and other environmental factors, making them exceptionally durable and suitable for use in extreme conditions.
These batteries are completely safe due to the addition of a thin metal coating that blocks any potentially harmful radiation. Additionally, as the decay of tritium yields stable helium-3, these batteries generate nuclear power without producing long-term environmentally harmful nuclear waste byproducts.

Limitations of Tritium-Powered Batteries
While tritium and betavoltaic batteries offer numerous advantages, they do have limitations that are closely tied to their benefits. One significant limitation is that as the tritium decays, the battery’s power output decreases over time. However, this reduction in output is gradual, predictable, and occurs over multiple decades, making tritium advantageous for microelectronic applications that require consistent power levels for extended periods of time. This long-term reduction in power supply can also be circumvented by simply supplying a device with extra tritium, ensuring an ample supply even after substantial decay.
While other isotopes have longer half-lives and can similarly provide power for extended periods, they are more radioactive than tritium, posing safety concerns for human applications and handling.
Tritium Battery Power Output
Over a 20-year lifespan, a NanoTritium™ battery will experience a decay in power output of approximately 68%. Initially, it will output its full value of 10 µW, dropping to around 5.674 µW after 10 years, and to roughly 3.219 µW after a full 20 years. This gradual decrease in output is characteristic of tritium betavoltaic batteries and is a result of the isotope’s radioactive decay.
This means that NanoTritum™ batteries could theoretically keep a device that only requires 3 µW to run fully powered for 20+ years.
Future Developments in Tritium Battery Technology
Original P100 series City Labs NanoTritium™ batteries have been used in laboratory and commercial settings since 2008 without needing replacement. Despite this success, the opportunities to further develop these batteries are numerous, and City Labs remains dedicated to remaining at the forefront of nuclear battery technology.
City Labs is currently developing the P200 series of NanoTritium™ batteries as the direct successor to the P100 line, optimized to provide more power and extended longevity across a range of applications. As we continue to expand the lifespan, performance, and power output of our NanoTritium™ batteries, we also aim to expand our partnerships as we develop new applications for these tritium-powered batteries to power a wider variety of devices across industries.


Check Out City Labs’ NanoTritium™ Battery
Read about our NanoTritium™ technology and see how it works. If you are looking for long-lasting, low-energy betavoltaic power sources to power your microelectronics, don’t hesitate to reach out to us. City Labs is always looking for new commercial partners to work with.
Contact Us Today




