Tritium, a rare isotope of hydrogen, serves as the power source behind our solid-state betavoltaic batteries. But why tritium? Why not one of the many other radioisotopes available for nuclear energy applications?
The answer lies in a careful balance of safety, efficiency, longevity, and regulatory feasibility—a balance that tritium strikes better than any other known isotope for ultra-low-power microelectronics.
In this article, we explore what makes tritium special, how it compares to other isotopes, and why it plays a central role in powering the future of long-term autonomous devices.
What Is Tritium?
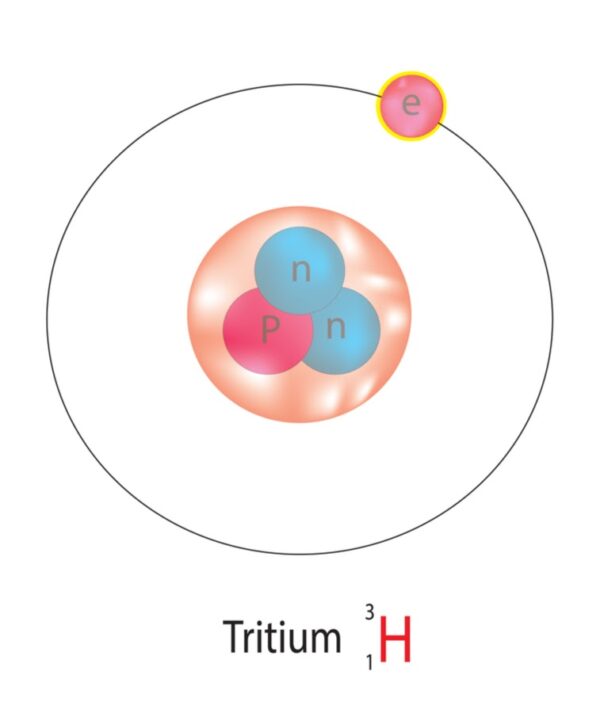
Tritium (³H) is a radioactive isotope of hydrogen. It has:
- One proton and two neutrons
- A half-life of 12.3 years
- A low-energy beta particle emission
In NanoTritium™ batteries, tritium undergoes beta decay, emitting electrons (beta particles) that strike a semiconductor junction and produce a small but continuous electric current—a process known as betavoltaic conversion. Because the decay is gradual and predictable, the battery provides consistent ultra-low power for decades without any need for recharging or maintenance.
Learn more about tritium and how it powers NanoTritium™
Comparing Tritium to Other Radioisotopes
Many radioisotopes, including plutonium-238, promethium-147, strontium-90, and nickel-63, have been used historically in nuclear batteries. Each has distinct characteristics, but most introduce one or more drawbacks that limit their use in small-scale, embedded, or human-adjacent systems.
Plutonium-238 (Pu-238)
- Half-life: ~87.7 years
- Radiation type: Alpha emitter
- Power density: Very high thermal output, ideal for radioisotope thermoelectric generators (RTGs)
- Benefits:
- Extremely long operational life
- Can generate enough heat to produce electricity via thermoelectric generators
- Proven in deep-space missions (e.g., Voyager, Curiosity Rover)
- Drawbacks:
- Highly radiotoxic, requiring heavy shielding
- Generates significant heat—unsuitable for compact electronics
- Tightly regulated, expensive, and primarily available only through government supply
Strontium-90 (Sr-90) / Cesium-137 (Cs-137)
- Half-lives: ~28.8 years (Sr-90), ~30.2 years (Cs-137)
- Radiation type: High-energy beta (Sr-90), beta and gamma (Cs-137)
- Power density: Moderate to high
- Benefits:
- Long lifespan with consistent output
- Readily available as a fission byproduct
- Historically used in marine and remote beacons
- Drawbacks:
- Emits penetrating radiation (gamma, high-energy beta)—requires thick shielding
- Environmental risks if containment fails
- Less suitable for embedded or human-adjacent systems
Promethium-147 (Pm-147)
- Half-life: ~2.6 years
- Radiation type: Low-energy beta emitter
- Power density: Low to moderate
- Benefits:
- Produces low-energy beta radiation with less shielding required
- Historically tested in betavoltaic designs
- Drawbacks:
- Short half-life limits lifespan of devices to just a few years
- Limited availability—production is complex and costly
- Not suitable for applications requiring multi-decade operation
Tritium (³H)
- Half-life: 12.3 years
- Radiation type: Very low-energy beta particles
- Power density: Low, but consistent
- Benefits:
- Extremely safe—beta particles cannot penetrate skin or paper
- Requires minimal shielding, allowing for compact, lightweight designs
- Decades-long output with no maintenance or degradation
- Non-heat-generating, ideal for sensitive microelectronics
- Easily sealed in solid-state form, reducing environmental and handling risks
- Drawbacks:
- Lower power output than some alternatives—best suited for ultra-low power devices, not high-consumption systems
Each radioisotope has its own strengths. But for applications that demand compact size, long-term reliability, low environmental risk, and zero maintenance, tritium offers the most practical and versatile solution.
Dive deeper into our nuclear battery technology
Why Tritium Is the Right Choice for Ultra-Low Power Applications
At City Labs, we didn’t just choose tritium for its radioactivity—we chose it for what it enables. Tritium makes it possible to build micro-scale, long-lasting batteries that are safe to deploy virtually anywhere.
Here’s what sets it apart in real-world use:
Safety Without Compromise
Tritium’s low-energy beta emissions mean it poses minimal health or environmental risks, even in the unlikely event of a breach. The tritium within each battery is stored within a metal hydride matrix and hermetically sealed, making these batteries extremely safe as they contain no moving parts, no pressurization, and no liquid electrolytes. This means that even if NanoTritium™ batteries are punctured or damaged, the tritium stored within is safely contained in a solid form.
No External Conditions Required
NanoTritium™ batteries work in total darkness, vacuum, radiation-heavy zones, or freezing temperatures. Unlike solar, thermal, or kinetic harvesters, they don’t rely on ambient energy—tritium’s decay is constant and autonomous.
Perfect for Long-Term, Low-Power Needs
Tritium’s half-life enables decade-plus power delivery at a consistent rate, gradually tapering off but never failing suddenly like a chemical battery. This reliability is critical for space missions, medical implants, remote sensors, and COMSEC systems.
A Small Isotope With Big Impact
Tritium may be one of the simplest atoms in nature, but its role in modern energy innovation is anything but basic. By leveraging tritium’s advantages, City Labs has developed a battery that works where others fail, lasts longer than most technologies, and enables a new class of resilient, autonomous systems.
In a world where reliability is everything—City Labs is committed to powering the future. If you’d like to partner with us on our journey, contact us today.